Pilkington Group
Professor Melanie Pilkington (FRSC)
Former Tier (II) Canada Research Chair
Department of Chemistry, Brock University
Research themes: Coordination chemistry, supramolecular chemistry, structural and solid state chemistry, molecular magnetism, organic conductors, bio-inorganic chemistry (MRI contrast agents).
Our research program involves the design and preparation of new molecule-based materials with interesting chemical, biological/medicinal and physical properties. Current projects are centered around ligand design particularly in the area of molecular magnetism, macrocyclic chemistry (crown ethers and Schiff-bases), organic conductors (organic field effect transistor devices) and medicinal inorganic chemistry (developing and studying a new family of MRI contrast agents).The general goals of our program are to develop novel molecular materials by: (i) developing rational synthetic routes to novel functional buidling blocks, (ii) understanding how molecular structure influences their physical properties and (iii) using these building blocks for the design and construction of new dual property materials or diagnostic agents. Molecular and supramolecular design principles pervade our thinking about the molecules we make, as do contemporary issues and challenges in materials science such as molecular magnetism, electrical conductivity, and nanotechnology.
The synthesis of new molecules lies at the heart of our research; most projects involve a challenging combination of organic, and coordination chemistry, we are also employing hydrothermal methodologies for the synthesis of frameworks. We also make use of a wide array of methods to study our compounds. In addition to the standard characterization techniques (NMR, MS, FT-IR, UV-Vis, IR, Raman, single crystal and powder X-ray crystallography, TGA etc.) we use other techniques to probe specific magnetic, electronic and optical properties such as electrochemistry, EPR and circular dichroism spectroscopy (CD), photoluminescence and magnetic susceptibility. We also employ ab initio computational methods, employing the MOLCAS programs to calculate the energy levels of the lanthanide ions in our single ion magnets as a complementary tool to our experimental studies. All of these methods can be performed "in house" and our lab is very well equipped for synthetic work including the preparation and handling of air-sensitive compounds. We have collaborations with scientists both within Canada and overseas to carry out more specialized studies such as state of the art imaging studies currently in progress at STTARR, located on the 7th floor of the MaRS tower in Toronto’s Medical Discovery District.
In the Pilkington group students synthesise new molecules and materials and study them employing the large variety of techniques listed above. We have projects to suite a wide range of expertise and interests including biological chemistry, organic synthesis, coordination chemistry, and molecular magnetism.
Read about our research highlighted here:
Below are examples of projects that are either in progress or recently completed:
Scientists have become the bearers of the torch of discovery in our quest for knowledge.
Stephen Hawking
4. The synthesis and characterization and study of new TTF derivatives
Since my Ph.D studies I have been interested in developing synthetic routes to functionalized tetrathiafulvalenes (TTFs) given their interesting electronic properties when either chemically or electrochemically oxidized. We started out developing routes to append stable organic (verdazyl) radicals onto the periphery of organosulfur donors working towards developing dual property magnetic conductors.
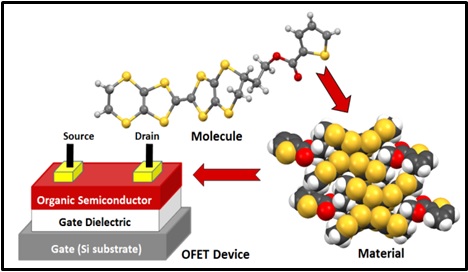
We are currently working on the preparation of new TTF and BEDT-TTF donors for incorporation into solution processible thin films for which we can evaluate their potential for applications as organic field effect transistors (OFETs). This work is carried out in collaboration with the Xerox Centre of Research in Canada (XRCC) which is based in Mississauga and involves a fair bit of organic synthesis. We prepare the thin films and carry out the OFET studies in collaboration with the molecular electronics group at Xerox. The first publication from this collaboration was recently published in Cryst. Eng. Comm. and a second has just been accepted for RSC Advances.
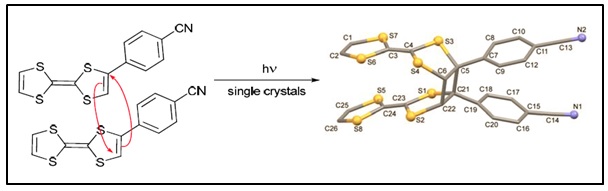
Since the crystal packing of these molecules in the solid state dictates their electronic properties we are also interested to study the molecular structures of these donors both when neutral or oxidized. Recently we published an unusual TTF donor that undergoes a [2 +2} photo-dimerization reaction in the single crystal which is quite rare.
Q. Wang, J.D. Wallis, Y. Wu and M. Pilkington, Cryst. Eng. Comm., 2014, 16, 7268.
J.J. Hayward, R. Gumbau-Brisa, C. Clarke, A. Alberola, J.M. Rawson and M. Pilkington, Cryst. Eng. Comm., 2014, 16, 7268.
2. Exploiting macrocyclic ligands for the synthesis and characterization of molecule-based magnets.
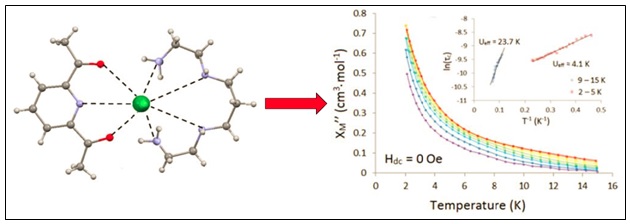
We have a number of macrocylic ligands that we are currently investigating. Recently published work includes studies on N5 and N3O2 Schiff-base systems. In 2007 we reported a covalently tethered dinuclear [Mn(N3O2)Cl(OH)2]2 macrocyclic building block and more recently we have demonstrated that the Co(II) derivative when co-crystallized with Fe(CN)6 can afford an interesting 1-D chain topology. We have begun to investigate Ln(III) complexes of Schiff-base macrocycles and recently reported the SMM properties of a Dy(IIII) complex which has an unusual pentagonal bipyramidal coordination geometry. We are investigating several different classes of macrocyclic ligands working towards the preparation and study of Ln(III)-based single molecule magnets (SMMs) as well as Fe(II) spin crossover complexes.
E.L. Gavey, Y. Beldjoudi, J.M. Rawson, T. Stamatatos and M. Pilkington, Chem. Commun., 2014, 50, 374.
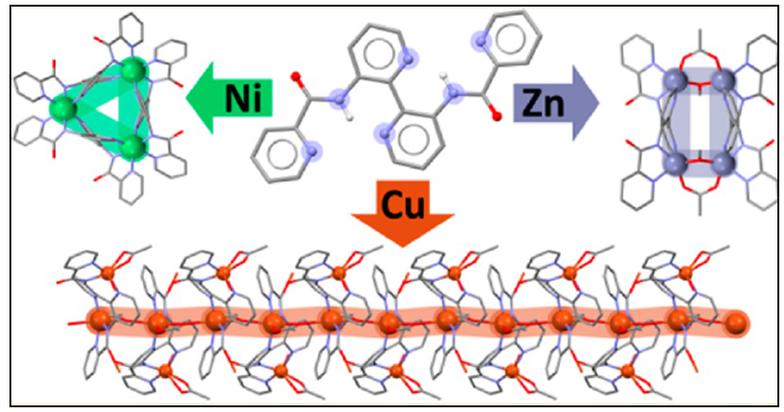
3. Ligand design for polynuclear, wheels, clusters and MOFs.
Optical microscopy image of crystals of a TTF derivative bridging microfabricated Au-electrodes
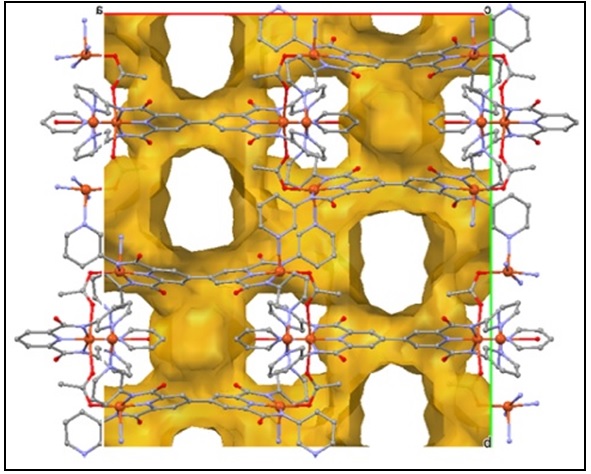
We are interested in preparing and exploring the coordination chemistry of large flexible 3,3'-disubstituted-2,2'-bipyridine and 4,4'-bipyridine ligands for the self-assembly of new topologies with interesting structural and physical properties. Following this methodology we have recently reported the self-assembly of dimers, trimers and clusters and we are in the process of characterizing a Cu(II) metal organic framework with a new structural topology.
N. Zararabi, J.J. Hayward, W. Clegg and M. Pilkington, Dalton, Trans., 2014, 2352.
A novel MOF structural topology, studies are in progress to fully characterize the physical properties of this material.
J. Regier and M. Pilkington, 2014, work in progress.
With our experience in ligand design and structural chemistry we have partnered with the Stamatatos group at Brock University. Utlizing Prof. Stamatatos's experience as one of the worlds most promising young magneto- cluster chemists we are working together on the preparation of new families of transition metal and/or lanthanide clusters. We are particularly interested in targeting the preparation of new systems with SMM properties that possess large energy barriers and/or dual property materials where the SMM properties are combined with a second physical property such as photoluminescence. Our first three collaborations have all been published as front covers, two in Chemical Communications and the third in Dalton Transactions.
1. Preparation and study of a new family of MRI contrast agents - working towards the next generation of dual property contrast media.
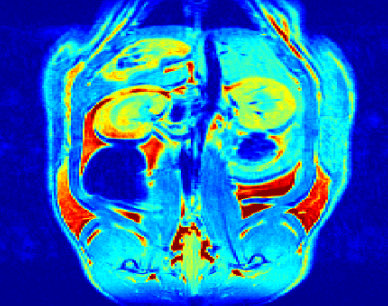
The objectives of this research program are to develop a new family of stable, target specific magnetic resonance imaging (MRI) contrast agents. Last year, over 1.5 million MRI scans were performed in Canada (Canadian Institute of Health Information), the majority of which involved contrast media. However, there are increasing concerns over the safety of the currently approved contrast agents; in addition, there is a push for the formation of compounds which can be localized to a given organ or tissue. The objectives of our research program are to address this challenge. We successfully prepared of a new family of MRI agents the first of which have been tested in vivo. Below is the MRI image of a rat that was injected with one of our agents. The view is through the kidneys 45 minutes after injection with our agent.
We are currently moving towards patenting our systems and looking for an industrial partner to develop this project further. The project involves, synthetic chemistry to prepare the ligands, coordination chemistry to prepare, purify and characterize the complexes and collaborative studies with researchers based at STTARR in Toronto to carry out in vivo toxicity and imaging studies on the agents. Below is the mini MRI used to image small animals at STTARR. To learn more watch Emma's 3MT presentation below:
MRI image - coronal slice through the kidneys 45 min after injection with one of our agents.
E.Gavey-Stares and M. Pilkington, 2014, work in progress.
N.J. Hurley, J. J. Hayward, J. M. Rawson, M. Murrie, and M. Pilkington, Inorg. Chem, 2014, 53, 8610.